Recruitment Begins in Phase 1 Trial of C-CAR088 for Multiple Myeloma
Written by |
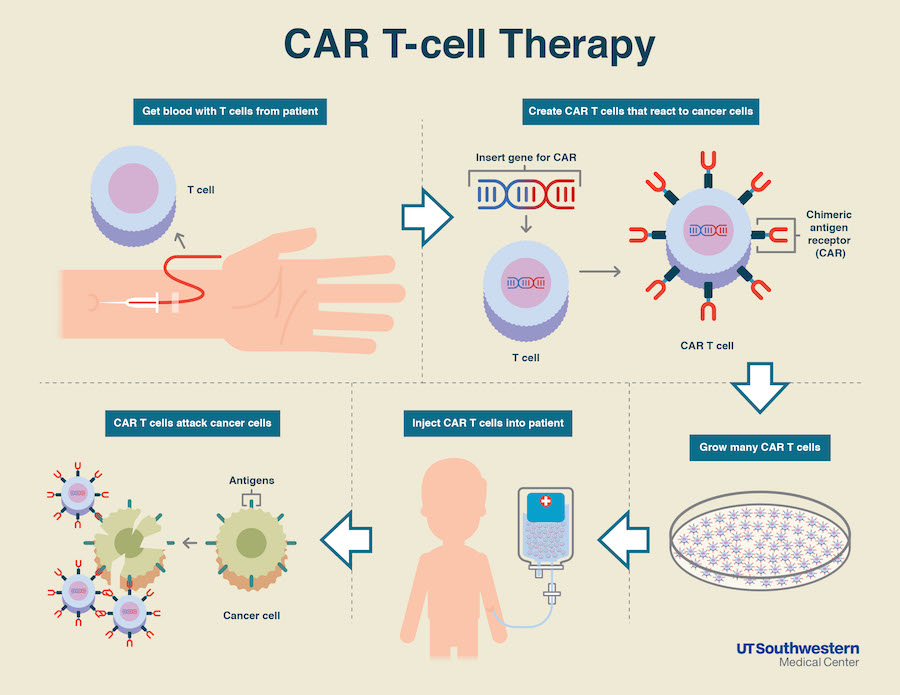
CAR-T Cell Infographic Credit: UT Southwestern
A Phase 1 trial in China is recruiting multiple myeloma patients who failed prior treatments to study Cellular Biomedicine’s CAR T-cell product, C-CAR088.
The trial is for patients who have received at least two multiple myeloma therapies — including a proteasome inhibitor such as Velcade (bortezomib) or Kyprolis (carfilzomib), or an immunomodulator such as Revlimid (lenalidomide) — or who received only one prior treatment but have no other treatment options for their condition.
CAR T-cells are immune T-cells collected from a patient’s own blood that are engineered to produce a chimeric antigen receptor (CAR) targeting a particular cancer molecule. In this case, C-CAR088 cells recognize a protein called B-cell maturation antigen, found at the surface of virtually all myeloma cells.
The T-cells are then expanded to millions and injected back into the patient, where they will be primed to identify and destroy tumor cells producing the BCMA factor.
The ongoing study will include 22 patients who will receive 1 million to 9 million C-CAR088 cells, given after a conditioning chemotherapy regimen. This chemotherapy is often given to make room for the new T-cells and kill any cancer cell that might remain in the body.
The trial’s main goal is to determine the treatment’s safety, measured through the incidence of adverse events. Secondary endpoints include overall response rate, the time to disease progression or death, the time CAR T-cells last in circulation, and changes in BCMA levels in the blood.
“China has seen a substantial increase in the incidence of multiple myeloma. As a drug development company, BCMA is the first of multiple assets that CBMG is advancing amongst our oncology pipeline beyond our collaboration with a global leader in cell therapy,” Tony (Bizuo) Liu, chief executive officer of Cellular Biomedicine, said in a press release.
In addition to C-CAR088, the company is also conducting preclinical studies for several other CAR T-cell therapies — one for CD22-positive acute lymphoblastic leukemia, one for CD20-positive non-Hodgkin’s lymphomas, and one that recognizes multiple targets in acute myeloid leukemia — a TCR T-cell product for liver cancer, and an approach that uses tumor infiltrating immune cells to fight non-small cell lung cancer.
“We hope to be able to provide expeditious, safe and effective therapies to cancer patients who currently have limited treatment options,” Lui said.



