Darzalex Faspro for multiple myeloma
What is Darzalex Faspro for multiple myeloma?
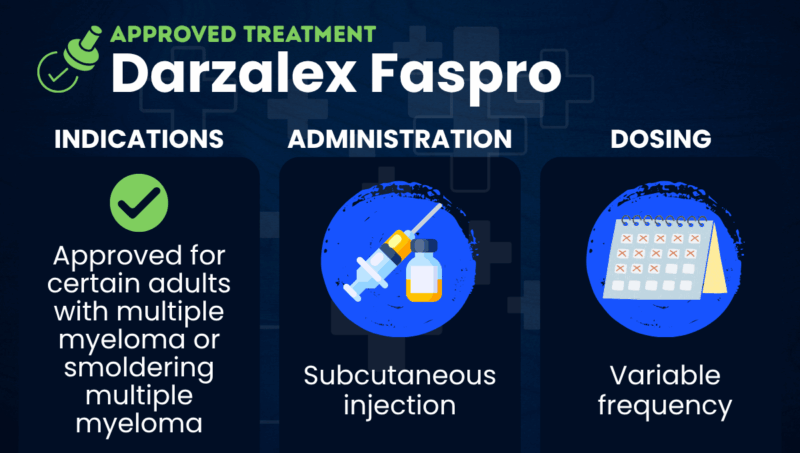
Darzalex Faspro (daratumumab and hyaluronidase-fihj) is an antibody-based therapy that’s approved for use as part of several treatment regimens for multiple myeloma and its asymptomatic precursor condition smoldering multiple myeloma (SMM).
The medication can be used for adults across various disease stages, including for SMM patients at a high risk of progression to active cancer. It’s also indicated for those newly diagnosed with multiple myeloma, and for people with relapsed or refractory multiple myeloma (RRMM), whose blood cancer has returned after initial treatment or failed to respond to it.
The main cancer-fighting ingredient in Darzalex Faspro is daratumumab, an antibody that binds to the CD38 protein in the body. That protein is found at high levels on cancerous myeloma cells, triggering their immune-mediated destruction. The other active ingredient is hyaluronidase, an enzyme that enables the medication to be formulated for under-the-skin (subcutaneous) injections.
Marketed by Johnson & Johnson, Darzalex Faspro is the first therapy to be approved in the U.S. for treating high-risk SMM. It’s also approved for treating certain individuals with light chain amyloidosis, a noncancerous disorder related to myeloma.
Johnson & Johnson also sells an into-the-vein (intravenous) formulation of daratumumab, called Darzalex, that’s approved in various multiple myeloma settings. By substantially shortening the treatment time from a few hours to a few minutes, Darzalex Faspro may offer more convenience than the intravenous version of the therapy.
Therapy snapshot
| Brand name | Darzalex Faspro |
| Chemical name | Daratumumab and hyaluronidase-fihj |
| Usage | Used to treat certain adults with multiple myeloma or smoldering multiple myeloma |
| Administration | Subcutaneous injection |
Who with multiple myeloma can take Darzalex Faspro?
Darzalex Faspro is approved in the U.S. for certain adults with multiple myeloma.
In newly diagnosed adults with multiple myeloma, Darzalex Faspro is approved to be used:
- with Velcade (bortezomib), Revlimid (lenalidomide), and dexamethasone as induction and consolidation therapy regardless of eligibility for an autologous stem cell transplant
- with Velcade, thalidomide, and dexamethasone in people who are transplant-eligible
- with Velcade, melphalan, and prednisone or with Revlimid and dexamethasone in transplant-ineligible patients
In adults with RRMM, Darzalex Faspro is approved to be used:
- with Revlimid and dexamethasone, or Velcade and dexamethasone, for people who have received at least one prior therapy
- with Pomalyst (pomalidomide) and dexamethasone in patients who have received at least one prior line of therapy that included Revlimid and a proteasome inhibitor (PI)
- with Kyprolis (carfilzomib) and dexamethasone in patients who have received one to three prior lines of therapy
- as a single therapy, or monotherapy, for patients who have received at least three prior lines of treatment, including a PI and an immunomodulatory agent (IMiD), and who are double-refractory to a PI and IMiD
Darzalex Faspro is also approved to be used as a monotherapy in adults with high-risk SMM.
It is contraindicated, or should not be used, in people with a history of serious immune reactions (hypersensitivity) to daratumumab, hyaluronidase, or any other ingredients in the medication.
The combination of Darzalex Faspro with Revlimid, thalidomide, or Pomalyst is contraindicated during pregnancy due to the risk of fetal harm or death.
How is Darzalex Faspro administered in multiple myeloma?
Darzalex Faspro is administered by a healthcare provider via subcutaneous injections into the abdomen, which take approximately 3-5 minutes. The recommended dose contains 1,800 mg of daratumumab.
Injection frequency varies by regimen. Generally, the medication will be administered once weekly for the first several doses, and then given every two to four weeks thereafter. Total treatment duration also varies. The medication may be given as follows:
- For RRMM and transplant-ineligible myeloma: Until disease progression occurs
- In newly diagnosed, transplant-eligible multiple myeloma: As a fixed number of doses before and after stem cell transplant
- For SMM: Until active myeloma is diagnosed or for a maximum of three years
Patients will be administered medications prior to receiving Darzalex Faspro to prevent potential side effects. Individuals receiving this therapy may also be sent home with oral steroid medications to take afterward.
Darzalex Faspro in multiple myeloma clinical trials
Darzalex Faspro’s approvals for active multiple myeloma were supported by data from a number of clinical trials:
- The Phase 3 COLUMBA trial (NCT03277105) demonstrated that Darzalex Faspro led to similar overall response rates — indicating the proportion of patients whose cancer shrinks or disappears — and survival rates as intravenous Darzalex among adults with RRMM who had received at least three previous lines of therapy.
- The Phase 2 PLEIADES trial (NCT03412565) generally showed that use of the subcutaneous therapy led to high treatment response rates and had similar clinical activity to intravenous Darzalex when used in combination with standard multiple myeloma regimens in both newly diagnosed and RRMM patient groups.
- The Phase 3 PERSEUS trial (NCT03710603) demonstrated that the addition of Dazalex Faspro to a standard treatment regimen used before and after stem cell transplant in individuals with newly diagnosed multiple myeloma led to higher treatment response rates and a lower risk of disease progression or death relative to standard care alone.
- The Phase 3 APOLLO trial (NCT03180736) showed that the addition of Darzalex Faspro to a standard regimen of Pomalyst and dexamethasone in certain RRMM patients lowered the risk of disease progression or death relative to the standard regimen alone.
Supportive data for some treatment regimens also came from previous clinical studies of intravenous Darzalex.
The Phase 3 AQUILA trial (NCT03301220), involving 390 patients, was the main study that supported Darzalex Faspro’s approval for high-risk SMM. The results showed that the subcutaneous therapy significantly lowered the risk of progression to active multiple myeloma or death compared with active monitoring without treatment.
Darzalex Faspro side effects
The exact side effect profile of Darzalex Faspro will depend on the regimen in which it is used. In general, possible side effects are:
- symptoms of nerve damage, such as numbness, tingling, or burning sensations
- fatigue
- swelling
- fever
- upper respiratory infections or pneumonia
- constipation or diarrhea
- nausea or vomiting
- musculoskeletal or back pain
- insomnia or other sleep issues
- rash
- cough
- muscle spasms
- shortness of breath
- high blood pressure
- headache
- injection site reactions
Darzalex Faspro may also lead to decreased blood cell counts. Patients should discuss with their healthcare providers exactly which side effects they might expect with their specific treatment regimen.
The therapy also comes with warnings that it could cause other potentially serious adverse events, such as:
- hypersensitivity reactions that could be life-threatening
- low counts of neutrophils, a type of infection-fighting immune cell, which could lead to serious or fatal infections
- low counts of platelets, involved in blood clotting after an injury
- harm to a developing fetus if used during pregnancy
Patients will be monitored for these events during treatment, and if they occur, Darzalex Faspro may need to be paused and appropriate treatment administered.
Female patients with productive potential should use an effective means of contraception while being treated with the medication and for three months after the last dose.
Rare Cancer News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 


