Blenrep (belantamab mafodotin-blmf) for multiple myeloma
Last updated Oct. 24, 2025, by Lindsey Shapiro, PhD

What is Blenrep for multiple myeloma?
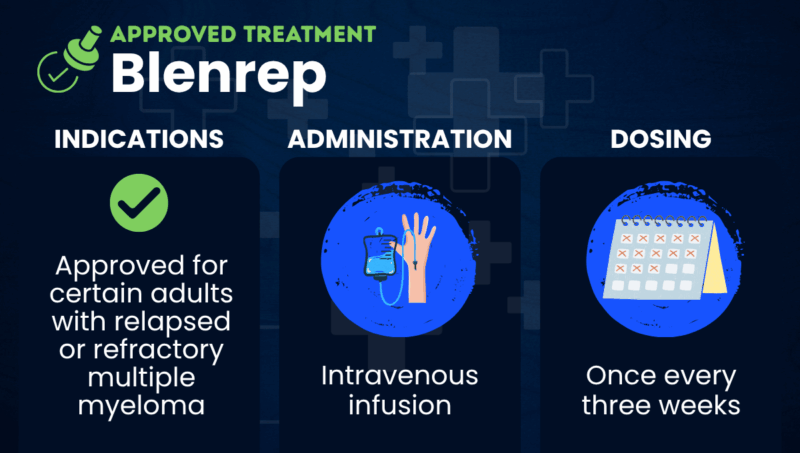
Blenrep (belantamab mafodotin-blmf) is an approved therapy that’s used to treat certain adults with multiple myeloma that has returned after (relapsed) or not responded to (refractory) other treatments.
Multiple myeloma is a blood cancer in which immune plasma cells grow out of control in the bone marrow.
Blenrep contains monomethyl auristatin F, a chemotherapy medication that kills cells by preventing them from replicating. It’s linked to an antibody that binds to the B-cell maturation antigen (BCMA) protein found on the surface of myeloma cells. The antibody brings the chemotherapy agent directly to cancer cells, killing them, while sparing other cells in the body.
Developed by GSK, the medication is given via intravenous, or into-the-vein, infusions. The company is also investigating Blenrep as an earlier line of treatment for certain newly diagnosed myeloma patients.
Therapy snapshot
| Brand name: | Blenrep |
| Chemical name: | Belantamab mafodotin-blmf |
| Usage: | Used to treat certain adults with relapsed or refractory multiple myeloma |
| Administration: | Intravenous infusion |
Who can take Blenrep?
Blenrep is approved in the U.S. as part of a combination regimen with Velcade (bortezomib) and dexamethasone to treat adults with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD).
While there are no contraindications for its use, Blenrep comes with a boxed warning that it may cause eye-related, or ocular, toxicity. For this reason, it is only available in the U.S. through a restricted access program.
The therapy is also approved as part of relapsed/refractory myeloma treatment regimens elsewhere, including in the European Union and Canada. In those areas, it can be used with Velcade and dexamethasone for patients who received at least one previous treatment, and in combination with Pomalyst (pomalidomide) and dexamethasone for people who received at least one prior treatment, including Revlimid (lenalidomide).
How is Blenrep administered?
Blenrep is given as an intravenous infusion by a healthcare provider once every three weeks. For the first eight cycles, it is administered in combination with bortezomib and dexamethasone. Thereafter, it is given alone until myeloma progresses or unacceptable toxicity occurs.
The recommended dose for each infusion is 2.5 milligrams per kilogram of body weight, although the dose may be modified if eye toxicity or other adverse reactions occur. The infusions take about 30 minutes to complete.
Blenrep in clinical trials
Blenrep’s U.S. approval was largely supported by data from the Phase 3 DREAMM-7 clinical trial (NCT04246047). That trial involved adults with relapsed or refractory multiple myeloma who had received at least one prior line of therapy and had experienced disease progression after the most recent one.
The participants were randomly assigned to receive Blenrep in combination with Velcade and dexamethasone, or a regimen of Darzalex (daratumumab), Velcade, and dexamethasone (DVd).
The results showed that among patients who matched the indication on the prescribing label:
- the risk of disease progression or death was reduced by nearly 70% with the Blenrep regimen compared with DVd, and the risk of death was approximately 50% lower
- the proportion of people achieving at least a partial treatment response or better was greater in the Blenrep group than in the DVd group (81.5% vs. 56.9%)
- more people on Blenrep than in the DVd group (23.1% vs. 2.8%) achieved minimal residual disease negativity, where there are no signs of the small number of cancer cells that can remain after treatment
Blenrep side effects
The most common side effects associated with Blenrep when used in combination with Velcade and dexamethasone are:
- eye-related changes
- upper respiratory tract infections
- liver toxicity
- diarrhea
- fatigue
- pneumonia, a lung infection
- COVID-19
Among the eye-related changes commonly reported with Blenrep were reduced visual acuity, blurred vision, dry eye, light sensitivity, eye irritation, and eye pain. Some individuals had a feeling like something was stuck in the eye. A clouding of the eye (cataracts) or other eye exam findings also occurred.
Blenrep also commonly causes blood test abnormalities, including reduced blood cell counts and high levels of liver enzymes.
According to the boxed warning, Blenrep causes changes in the eyes that can severely impair vision or lead to symptoms such as blurred vision and dry eyes. As such:
- Patients must receive eye exams from an eye care professional before starting treatment, before receiving each dose, any time they experience new or worsening symptoms, and as clinically indicated.
- Patients should promptly alert their physician if any eye changes occur, as a dose modification may be needed. Blenrep may need to be temporarily paused or permanently discontinued, depending on the severity.
- Patients are advised to use preservative-free artificial tears at least four times a day throughout Blenrep treatment. Contact lenses should be avoided, with the exception of bandage contact lenses used under the direction of an eye care professional.
Blenrep may also cause other potential serious side effects, including:
- low counts of platelets, which are involved in blood clotting
- harm to a developing fetus if used during pregnancy
Women with reproductive potential should use effective contraception during treatment with Blenrep and for four months after the last dose. Likewise, men who have female partners with reproductive potential should use effective contraception during treatment and for six months after the last dose.
Rare Cancer News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
